Mintezol Prescribing Information
Package insert / product label
Generic name: thiabendazole
Dosage form: Chewable Tablets and Suspension
Drug class: Anthelmintics
Medically reviewed by Drugs.com. Last updated on Mar 25, 2024.
On This Page
Mintezol Description
MINTEZOL1 (Thiabendazole) is an anthelmintic provided as 500 mg chewable tablets, and as a suspension, containing 500 mg thiabendazole per 5 mL. The suspension also contains sorbic acid 0.1% added as a preservative. Inactive ingredients in the tablets are acacia, calcium phosphate, flavors, lactose, magnesium stearate, mannitol, methylcellulose, and sodium saccharin. Inactive ingredients in the suspension are an antifoam agent, flavors, polysorbate, purified water, sorbitol solution, and tragacanth.
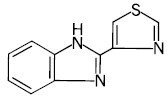
Thiabendazole is a white to off-white odorless powder with a molecular weight of 201.26, which is practically insoluble in water but readily soluble in dilute acid and alkali. Its chemical name is 2-(4-thiazolyl)-1H -benzimidazole. The empirical formula is C10H7N3S and the structural formula is:
- 1
- Registered trademark of MERCK & CO., Inc. COPYRIGHT © MERCK & CO., Inc., 1983 All rights reserved
Mintezol - Clinical Pharmacology
In man, thiabendazole is rapidly absorbed and peak plasma concentration is reached within 1 to 2 hours after the oral administration of a suspension. It is metabolized almost completely to the 5-hydroxy form which appears in the urine as glucuronide or sulfate conjugates. In 48 hours, about 5% of the administered dose is recovered from the feces and about 90% from the urine. Most is excreted in the first 24 hours.
Mechanism of Action
The precise mode of action of thiabendazole on the parasite is unknown, but it may inhibit the helminth-specific enzyme fumarate reductase.
Thiabendazole is vermicidal and/or vermifugal against Ascaris lumbricoides (“common roundworm”), Strongyloides stercoralis (threadworm), Necator americanus, and Ancylostoma duodenale (hookworm), Trichuris trichiura (whipworm), Ancylostoma braziliense (dog and cat hookworm), Toxocara canis and Toxocara cati (ascarids), and Enterobius vermicularis (pinworm).
Its effect on larvae of Trichinella spiralis that have migrated to muscle is questionable.
Thiabendazole also suppresses egg and/or larval production and may inhibit the subsequent development of those eggs or larvae which are passed in the feces.
Related/similar drugs
metronidazole, Flagyl, ivermectin, albendazole, mebendazole, nitazoxanide, pyrantel
Indications and Usage for Mintezol
MINTEZOL is indicated for the treatment of:
-
Strongyloidiasis (threadworm)
-
Cutaneous larva migrans (creeping eruption)
-
Visceral larva migrans
Trichinosis: Relief of symptoms and fever and a reduction of eosinophilia have followed the use of MINTEZOL during the invasion stage of the disease.
Thiabendazole is usually inappropriate as first line therapy for enterobiasis (pinworm). However, when enterobiasis occurs with any of the conditions listed above, additional therapy is not required for most patients.
MINTEZOL should be used only in the following infestations when more specific therapy is not available or cannot be used or when further therapy with a second agent is desirable: Uncinariasis (hookworm: Necator americanus and Ancylostoma duodenale); Trichuriasis (whipworm); Ascariasis (large roundworm).
Contraindications
Hypersensitivity to this product.
Thiabendazole is contraindicated as prophylactic treatment for pinworm infestation.
Warnings
If hypersensitivity reactions occur, the drug should be discontinued immediately and not be resumed. Erythema multiforme has been associated with thiabendazole therapy; in severe cases (Stevens-Johnson syndrome), fatalities have occurred.
Because CNS side effects may occur quite frequently, activities requiring mental alertness should be avoided.
Jaundice, cholestasis, and parenchymal liver damage have been reported in patients treated with MINTEZOL. In rare cases, liver damage has been severe and has led to irreversible hepatic failure. (See ADVERSE REACTIONS.)
Abnormal sensation in eyes, xanthopsia, blurred vision, drying of mucous membranes, and Sicca syndrome have been reported in patients treated with MINTEZOL. These adverse effects of the eye were in some cases persistent for prolonged intervals which have exceeded one year. (See ADVERSE REACTIONS.)
Thiabendazole should not usually be used as first line therapy for the treatment of enterobiasis. It should be reserved for use in patients who have experienced allergic reactions, or resistance to other treatments.
Precautions
General
MINTEZOL is not suitable for the treatment of mixed infections with ascaris because it may cause these worms to migrate.
Ideally, supportive therapy is indicated for anemic, dehydrated or malnourished patients prior to initiation of the anthelmintic therapy.
In the presence of hepatic or renal dysfunction, patients should be carefully monitored.
MINTEZOL should be used only in patients in whom susceptible worm infestation has been diagnosedand should not be used prophylactically.
Information for Patients
Because CNS side effects may occur quite frequently, activities requiring mental alertness should be avoided.
Laboratory Tests
Rarely, a transient rise in liver function tests has occurred in patients receiving MINTEZOL.
Drug Interactions
Thiabendazole may compete with other drugs, such as theophylline, for sites of metabolism in the liver, thus elevating the serum levels of such compounds to potentially toxic levels. Therefore, when concomitant use of thiabendazole and xanthine derivatives is anticipated, it may be necessary to monitor blood levels and/or reduce the dosage of such compounds. Such concomitant use should be administered under careful medical supervision.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Thiabendazole has been used in numerous short- and long-term studies in animals at doses up to 15 times the usual human dose and was without carcinogenic effects. It did not adversely affect fertility in the mouse at 2½ times the usual human dose or in the rat at a dose equivalent to the usual human dose. Thiabendazole had no mutagenic activity in in vitro microbial mutagen test, the micronucleus test and the host mediated assay in vivo.
Pregnancy
Pregnancy Category C: Reproduction and teratogenic studies done in the rabbit at a dose up to 15 times the usual human dose, in the rat at a dose equivalent to the human dose, and in the mouse at a dose up to 2½ times the usual human dose, revealed no evidence of harm to the fetus. In an additional study in the mouse, no defects were observed when thiabendazole was given in aqueous suspension, at a dose 10 times the usual human dose; however, cleft palate and axial skeletal defects were observed when thiabendazole was suspendedin olive oil and given at the same dose. There are no adequate and well controlled studies in pregnant women. MINTEZOL should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because of the potential for serious adverse reactions in nursing infants from MINTEZOL, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
The safety and effectiveness of thiabendazole for the treatment of Strongyloidiasis, Ascariasis, Uncinariasis, Trichuriasis and Trichinosis in pediatric patients weighing less than 30 lbs has been limited.
Geriatric Use
Clinical studies of MINTEZOL did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
This drug is metabolized almost completely by the liver, and the metabolites are known to be substantially excreted by the kidney, therefore the risk of toxicity may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
Adverse Reactions/Side Effects
Gastrointestinal: anorexia, nausea, vomiting, diarrhea, epigastric distress, abdominal pain, jaundice, cholestasis, parenchymal liver damage and hepatic failure. (See WARNINGS.)
Central Nervous System: dizziness, weariness, drowsiness, giddiness, headache, numbness, hyperirritability, convulsions, collapse, confusion, depression, floating sensation, weakness and lack of coordination.
Special Senses: tinnitus, abnormal sensation in eyes, xanthopsia, blurred vision, reduced vision, drying of mucous membranes (mouth, eyes, etc.), Sicca syndrome. (See WARNINGS.)
Cardiovascular: hypotension.
Metabolic: hyperglycemia.
Hematologic: transient leukopenia.
Genitourinary: hematuria, enuresis, malodor of the urine, crystalluria.
Hypersensitivity: pruritus, fever, facial flush, chills, conjunctival injection, angioedema, anaphylaxis, skin rashes (including perianal), erythema multiforme (including Stevens-Johnson syndrome), and lymphadenopathy.
Miscellaneous: appearance of live Ascaris in the mouth and nose.
Overdosage
Overdosage may be associated with transient disturbances of vision and psychic alterations.
There is no specific antidote in the event of overdosage. Therefore, symptomatic and supportive measures should be employed. Emesis should be induced or gastric lavage performed carefully.
The oral LD50 of MINTEZOL is 3.6 g/kg, 3.1 g/kg and 3.8 g/kg in the mouse, rat, and rabbit, respectively.
Mintezol Dosage and Administration
The recommended maximum daily dose of MINTEZOL is 3 grams.
MINTEZOL should be given after meals if possible. Tablets MINTEZOL should be chewed before swallowing. Dietary restriction, complementary medications and cleansing enemas are not needed.
The usual dosage schedule for all conditions is two doses per day. The dosage is determined by the patient's weight.
A weight-dose chart follows:
| Weight | Each Dose | |
| g | mL | |
| 30 lb | 0.25 (½ tablet) | 2.5 (½ teaspoon) |
| 50 lb | 0.5 (1 tablet) | 5.0 (1 teaspoon) |
| 75 lb | 0.75 (1½ tablets) | 7.5 (1½ teaspoons) |
| 100 lb | 1.0 (2 tablets) | 10.0 (2 teaspoons) |
| 125 lb | 1.25 (2½ tablets) | 12.5 (2½ teaspoons) |
| 150 lb & over | 1.5 (3 tablets) | 15.0 (3 teaspoons) |
The regimen for each indication follows:
| Therapeutic Regimens | ||
| Indication | Regimen | Comments |
|
||
| *STRONGYLOIDIASIS | 2 doses per day for 2 successive days. | A single dose of 20 mg/lb or 50 mg/kg may be employed as an alternative schedule, but a higher incidence of side effects should be expected. |
| CUTANEOUS LARVA MIGRANS (Creeping Eruption) | 2 doses per day for 2 successive days. | If active lesions are still present 2 days after completion of therapy, a second course is recommended. |
| VISCERAL LARVA MIGRANS | 2 doses per day for 7 successive days. | Safety and efficacy data on the seven-day treatment course are limited. |
| *TRICHINOSIS | 2 doses per day for 2-4 successive days according to the response of the patient. | The optimal dosage for the treatment of trichinosis has not been established. |
| Other Indications
*Intestinal roundworms (including Ascariasis, Uncinariasis and Trichuriasis) | 2 doses per day for 2 successive days. | A single dose of 20 mg/lb or 50 mg/kg may be employed as an alternative schedule, but a higher incidence of side effects should be expected. |
How is Mintezol supplied
No. 3331 — MINTEZOL Suspension, 500 mg per 5 mL, is white to off-white and is supplied as follows:
NDC 0006-3331-60 in bottles of 120 mL
(6505-00-935-5835, 0.5 g/5 mL, 120 mL).
Storage
Store in a well-closed container at controlled room temperature [15-30°C (59-86°F)]. Protect from freezing.
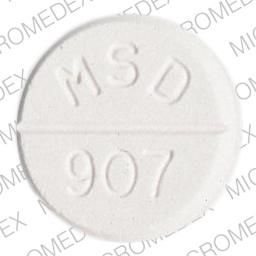
No. 3332 — MINTEZOL Chewable Tablets, 500 mg, are white to off-white, orange-flavored, round, scored, compressed tablets, coded MSD 907 on one side and MINTEZOL on the other.
They are supplied as follows:
NDC 0006-0907-36 unit dose packages of 36
(6505-01-226-9909, 500 mg chewable, individually sealed 36's).
| MINTEZOL
thiabendazole tablet, chewable |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| MINTEZOL
thiabendazole suspension |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Merck & Co., Inc. |
More about Mintezol (thiabendazole)
- Check interactions
- Compare alternatives
- Drug images
- Side effects
- Dosage information
- During pregnancy
- Drug class: anthelmintics